New standards and test methods are needed for medical textiles.
by Matthew Hardwick
The spread of infection is a concern for all of us and in many circumstances, but nowhere is it more concerning than in health care. The spread of infection both to and from medical environments is an ever-present problem in hospitals and clinics around the world. Numerous resources are being dedicated to fight the war against hospital-acquired infections. Healthcare textiles, including scrubs, lab coats and linens, are at the front line of this war against infected body fluids and the spread of disease.
Healthcare workers, primarily nurses, are in constant contact with patients. When checking a patient’s vitals or wound dressing, their scrubs are in direct contact with the patient’s skin. But nurses see many patients throughout the day and rarely change their scrubs—generally only when visibly soiled. Therefore, they may become a walking source of infectious material. Patient beds, sheets and blankets, with their intimate patient contact, are changed frequently, but they’re pulled off of the bed and may spread pathogens through the air during changing, or through contact with other linens and equipment during the changing process. Even privacy curtains present a danger for the spread of infections and have been linked to several disease outbreaks[1][2][3].
The textile world understands the potential role of textiles in preventing the spread of infections. Antimicrobial active ingredients such as copper, silver, zinc and quaternary ammoniums have been added to a variety of textiles, including bed linens, healthcare worker uniforms, and privacy curtains, in order to reduce microbial contamination of healthcare textiles. Further, other agents such as chlorine-binding and hydrophobic (fluid-resistant) chemistries have been added to these textiles to reduce the problem, as well.
In healthcare, there is little question that textiles act as mobile vectors of infectious microorganisms[4]; however, there is considerable controversy about how effective microbe-reducing textiles are at actually combating the spread of microbes[5][6]. This controversy stems from how we determine antimicrobial efficacy of textiles and how that translates to the medical world.
Antibacterial textile testing
The first topic to address should be the terms “antibacterial” versus “antimicrobial.” Antimicrobial implies that a technology works against all microbes, including bacteria and fungi (and viruses to a lesser extent). Much of the testing performed on “antimicrobial” textiles in healthcare is actually antibacterial. While fungi, such as Candidaspecies, are important in healthcare, bacterial species, such as Staphylococcus aureus (S. aureus) and Escherichia coli(E. coli), are far more prevalent.
While I have used antimicrobial to describe these chemistries (indeed, they are, generally, effective against bacteria and fungi, and viruses, in some instances), healthcare textiles are generally tested for efficacy against bacteria. Therefore, I will refer to healthcare textile testing as antibacterial from this point on and limit testing options only to testing antibacterial chemistries.
Testing laboratories have several means to test antibacterial textiles (Table 1). In the United States, both the American Association of Textile Chemists and Colorists (AATCC) and ASTM International have developed antibacterial textile tests. Internationally, there are several available with the most prominent coming from the International Standards Organization (ISO) and the Japanese Industrial Standards (JIS) from the Japanese Industrial Standards Committee.
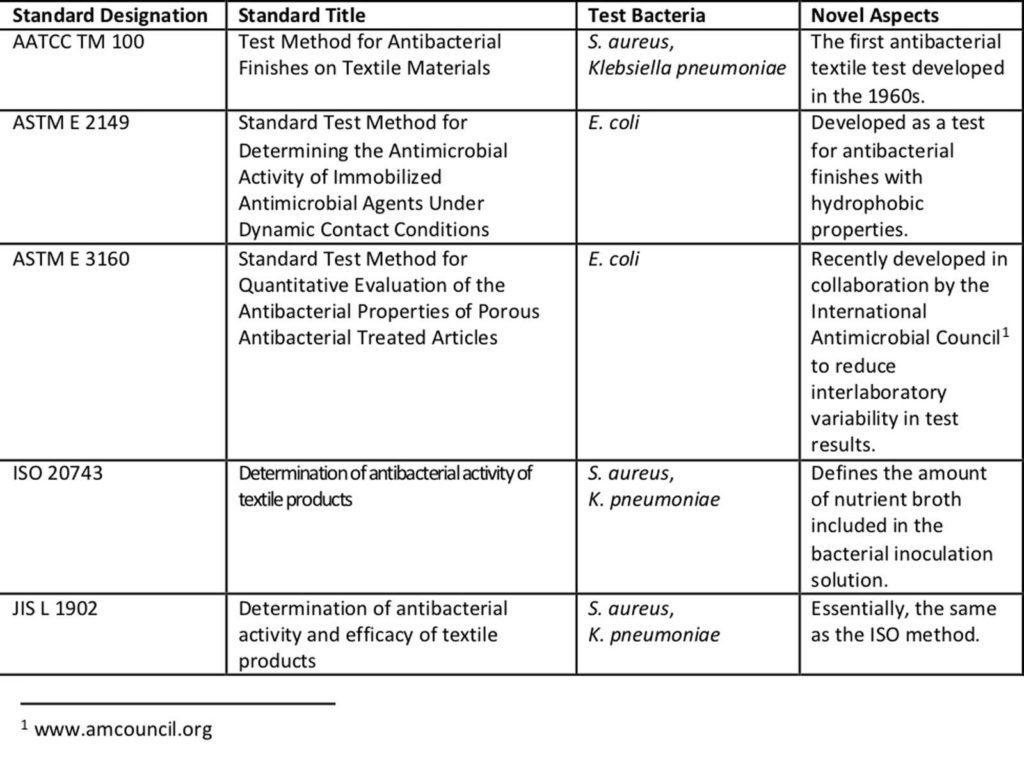
This battery of tests does a great job at describing the capacity of an antibacterial textile to either prevent bacterial growth or kill the specified bacteria. These methods are excellent for refining the antibacterial formulation in a textile product during development and for determining quality of antibacterial application during production. However, they fall well short when it comes to determining if an antibacterial textile will have an impact on bacteria in the real world.
Current methods
While each of the established test methods described in Table 1 serves an important role in the development of effective antibacterial textiles, they fall short with regards to the medical community. Much of a lack of relevance has to do with the conditions of the methods themselves. With the exception ASTM E 2149, each of these tests has a 24-hour contact time between a liquid bacterial inoculum and the treated textile. Essentially, the textile is kept in a humid chamber with the bacteria. While humid conditions are critical for the bacteria (they will die in a dry environment) and a 24-hour period is needed to see adequate growth of the bacteria on an untreated textile, these conditions do not represent real life for a medical textile, such as a typical scrub suit (unless it’s at the bottom of a hamper).
With ASTM E 2149 the testing conditions are even more convoluted. Because this method was developed for hydrophobic textiles, like those treated with quaternary silanes, the liquid bacterial inoculum must be in constant and vigorous contact with a treated textile. Therefore, a greater inoculum volume is used and the system is shaken over the course of an hour using a wrist-action shaker. Does this remind you of anything your clothing experiences during the course of a day? Of course not, it is highly contrived and artificial, mainly because it was intended to be used as a fast, quality assurance test method.
Indeed, there is still considerable controversy with regards to clinical evidence for antibacterial efficacy of treated textiles. The lack of a solid connection between laboratory data and clinical performance may be due to the nature of the test methods as described above. As you can imagine, clinical trials, much like field wear trials, are very complicated tasks involving numerous personnel and, at times, departments. They are, therefore, extremely expensive and time consuming. The industry needs test methods that bridge the gap between the basic test methods currently available and arduous and expensive clinical trials.
What is needed
The Expert Group on Efficacy of Biocide Treated Articles (EBTA) within the Organisation for Economic Co-operation and Development (OECD), Task Force on Biocides recommends a three-tiered approach to determining efficacy of antibacterial treated articles[7][8].
- Tier 1: Proof of Principle defines the basic industry standard tests that determine efficacy of an antibacterial treated article in a controlled laboratory environment.
- Tier 2: Simulated Use defines the translational tests that mimic end-use scenarios, environments and incubation times.
- Tier 3: In-Use Evaluation substantiates direct health benefit claims or supports marketing initiatives by the development of clinical trials that take place in end-use environments under real-world conditions.
The current standard test methods (Table 1) fit the Tier 1 category. Clearly, clinical trials fall into Tier 3 testing. What are sorely missing are Tier 2 test methods for medical textiles. Tier 2 methods should be laboratory-based and predictive of eventual clinical trials. Such an approach will, in the end, save the industry considerable time and money.
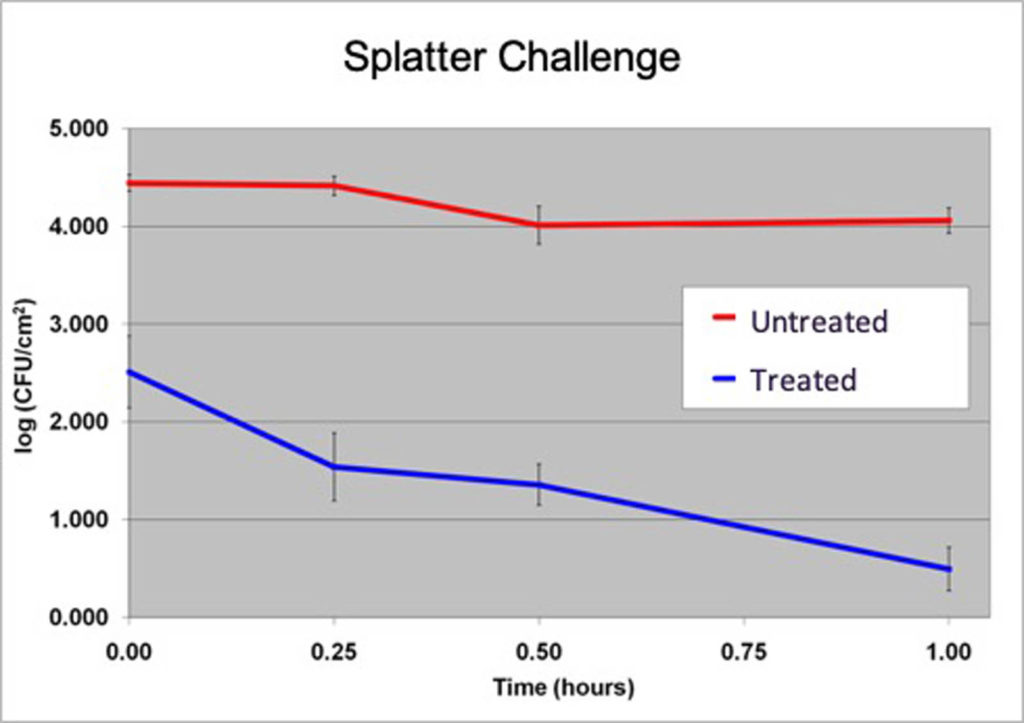
ResInnova Laboratories is pioneering Tier 2 testing in the medical textile field. The company has developed the fabric challenge assays[9] in response to this lack of predictive testing in the antibacterial textile field. These assays introduce microbes to textile swatches via aerosol, direct contact and splatter inoculation (Figure 1), mimicking how medical textiles become contaminated in real life.

The assays have the ability to differentiate products based on how they perform against each of the three inoculation techniques. Moreover, the results, especially for the splatter assay (Figure 2), are predictive of clinical trial performance. The textile used for the development of the fabric challenge assays is the same as what was used to show a significant reduction in methicillin-resistant S. aureus (MRSA) contamination in the Bearman cross-over trial.
A path forward
Antimicrobial textiles have been around for a long time, and testing the efficacy of these textiles has been around almost as long. However, very little has changed in the testing world since the original methods were developed. The link between effectiveness shown with these methods run in the laboratory and real-world efficacy is simply inadequate.
The tiered approach to testing these textiles is extremely important and should guide the industry as it moves forward into newer industries, such as the medical field, where scrutiny and regulation are more stringent. However, to date, there are no available Tier 2 testing standards for medical textiles. While Ressinova Laboratories has developed a series of methods, the fabric challenge assays, they have not become standards. It is time for the industry to rise to this challenge and bring forward a new era of antibacterial testing.
Matthew Hardwick, Ph.D. is president and CEO of Resinnova Laboratories, Silver Spring, Md. The company offers a range of services to help clients’ facilities improve environmental cleaning. ResInnova’s Laboratory tests products that reduce pathogens on environmental surfaces, including textiles. www.resinnovalabs.com.
Figure 1 and 2, Table 1 provided by Ressinova Laboratories.
FOOTNOTES
[1] Ohi M, et al., Am J Infect Control. 2012.
[2] Das I, et al. J Hosp Infect. 2002.
[3] Mahida N, et al. J Hosp Infect. 2014.
[4] Burden, M et al., J Hosp Med. 2011. 6(4):177-182.
[5] Burden, M et al., J Hosp Med. 2013. 8(7):380-385.
[6] Bearman, GM et al., Infect Control Hosp Epidemiol. 2012. 33(3): 268-275.
[7] OECD (2008). Guidance Document on the Evaluation of the Efficacy of Antimicrobial Treated Articles with Claims for External Effects. ENV/JM/MONO(2008)27. Environment, Health and Safety Publications, series on Biocides, No. 1, OECD, Paris, 2008.
[8] OECD (2007). Analysis and assessment of current protocols to develop harmonised test methods and relevant performance standards for the efficacy testing of treated articles/treated materials. ENV/JM/MONO(2007)4.
[9] https://www.astm.org/DIGITAL_LIBRARY/STP/PAGES/STP155820120184.htm
 TEXTILES.ORG
TEXTILES.ORG


