
PLA, or polylactic acid, is a relatively recent innovation, but it is already the most commonly used bioplastic today. It’s a polyester, but with a key difference: instead of being made from petrochemicals, it’s plant-sourced. The process, developed by Minnetonka, Minn.-based Cargill Inc., involves fermenting readily available raw materials such as corn, rice or sugarcane into lactic acid, then polymerizing the lactic acid to make plastic pellets. From there, the plastic can be manufactured into items such as car interiors, cold-drink cups and food or cosmetic packaging, or it can be made into nonwoven fabric by means of meltblowing or spunbonding.
Nonwoven PLA has been most commonly used in agricultural fabrics and geotextiles. The appeal is obvious: PLA boast a smaller environmental footprint than petroleum-based plastic, and it’s readily biodegradable. Depending on the exact formulation of the product and where it’s installed and/or discarded, it generally begins to degrade in about a year.
The same characteristics that make PLA well suited for agricultural and geotechnical fabrics also make it useful for healthcare applications. Early on, scientists realized the potential for PLA—which degrades over time into lactic acid, a compound that is naturally present in the body—for medical implants such as anchors, screws, pins, rods and meshes. These products break down inside the body within six months to two years, gradually transferring their load to the bone as the area heals.
Hospitals and other medical care facilities use an array of disposable paper and fabric items in order to reduce the transmission of bacteria, viruses and other pathogens. If so much material must be thrown away, it is desirable for the material to be biodegradable.
Rethinking disposables
“One of the big issues [in health care] is folks picking up bugs when they’re at the hospital getting treatment,” says Robert Green, global business leader for Ingeo fibers and nonwovens at NatureWorks LLC, Minnetonka, Minn. “Just by its nature, the use of nonwovens is a great way to mitigate that. But we also think that since these products are disposable, using a product that has a smaller environmental footprint to basically any of the other options that are in commercial use today is a good proposition.”
Green says nonwoven PLA is an excellent fit for disposable textile components such as medical drapes and gowns. It bridges the gap between performance and disposability, he says—neither must be sacrificed in the name of the other.
Keri Seitz, vice president of sales and customer relations at Biovation LLC, Boothbay, Maine, agrees. “There is a possibility for sterile drapes, for example, or barrier materials,” she says. “We’ve been talking about dental bibs, too, and personal protective equipment. If you think about where paper-based products are used now within the healthcare industry, I think that PLA can meet or exceed the performance in health care for those similar products.”
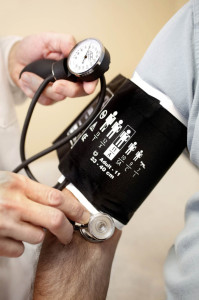
One of the products Biovation has already brought to market is a PLA blood pressure cuff shield. It’s approved for use in Europe and Canada, and is awaiting FDA approval for sale in the United States. “In theory, each blood pressure cuff gets disinfected prior to being used on the next patient,” she explains. “In practice, that doesn’t happen very often.
“There’ve been multiple studies done that have shown that the blood pressure cuff can be a vector for bacteria and other organisms from one patient to another, so our goal with the product was to create something that could act as a barrier from patient to patient to prevent the transmission of bacteria. And then we embedded it with antimicrobials, consisting of a silver and copper additive, to actually kill them on contact before the next patient uses the product.”
Biovation is also developing PLA flushable and disposable wipes. Seitz says currently available wipes wreak havoc on sewage treatment systems because, although they’re made of biodegradable materials, performance-related additives hamper that degradability. PLA has inherent strength and other characteristics that remove the necessity for these additives.
Direct-contact products
Green says there’s another part of the healthcare space that’s an excellent fit for nonwoven PLA: wound care products and other applications where material is in direct contact with the skin.
“Studies have shown that our material is very comfortable in direct skin contact applications because it has very good inherent moisture management properties,” he says. “It breathes and it doesn’t pick up odors. It breaks down to lactic acid, which is a natural product that we all have in our bodies. But also, lactic acid is used a lot in food applications as a preservative. Bacteria don’t proliferate on PLA products; it has natural, mild antimicrobial type properties that we think will drive a lot of interest and opportunities in the hygiene space.”
These attributes are a big selling point for wound care dressings that are being developed at Biovation, says Seitz. And the company has also developed a PLA boot-drying product for the U.S. Marine Corps that may have significant implications for healthcare applications in which fungal infections must be reduced or prevented.
Green says scientists at the Nonwovens Institute at North Carolina State University are doing a great deal of work around PLA in wound care and other related applications. In addition, he says PLA may have a future role in feminine hygiene products. Because the natural flora of the area are mostly Lactobacillus bacteria that produce lactic acid, PLA may be one of the most “friendly” materials to use.
Machinery already in place
The most significant challenge for PLA in healthcare applications is the price of the material. Seitz says PLA can be 50 percent more expensive, or even higher, than cheap petroleum-based nonwovens manufactured overseas. Because of that price gap, it’s currently crucial that PLA be selected for applications in which its inherent qualities are a significant advantage: either the environmental advantages of the product must be highly valued, or the microbial-resistance and moisture management attributes must be central to the product’s success.
But economics change over time. As petroleum becomes more rare, the price gap will begin to close, and in the shorter term, increased efficiency and volume in the PLA supply chain will make a big dent in the difference.
“Just as you see with everything else, anything new is typically run at smaller scale,” says Green. “Companies that are involved with it tend to look for higher margins on those new products. But if we continue to drive innovation with our material and facilitate growth, economies of scale throughout those supply chains can be realized. That’s what we think it’s going to take to fully address that cost issue.”
Nonwovens processes lend themselves to efficient, short supply chains, he says, because the processes are less complex than those of woven fabrics. “When you think about things like spunbond or meltblown, it’s directly from chip to fabric, so it’s fewer steps to produce those products,” he says. “So that makes the task easier. But we need to educate folks about the best applications for this material so we can realize continued growth and economies of scale.”
One of the biggest things in favor of PLA nonwovens for the healthcare market may be the ease of manufacturing the bio-based material. Green has worked extensively with colleagues at Troisdorf, Germany-based Reifenhäuser-Reicofil, Biax-Fiberfilm Corp. of Greenville, Wis., and other companies that produce nonwoven manufacturing machinery, and has found that the differences between manufacturing PLA and synthetic nonwovens are very small.
“We’ve had a good opportunity to work with the equipment manufacturers and educate them on what it takes to process our material,” he says. “Generally speaking, what we’ve found is that most of the traditional equipment that’s used in fiber processing works quite well. Our material can be processed on most equipment, with typically minor process adjustments or minor equipment configuration changes. Anything new, folks have to learn—but we’re impressed with the progress that we’re seeing.”
Jamie Swedberg is a freelance writer.
 TEXTILES.ORG
TEXTILES.ORG


